shelf life calculator for pharmaceutical products
This online service helps you to know how long your product is in good condition. A pharmaceutical product is typically manufactured in batches.
Stability Testing And Shelf Life Estimation
Shelf-Life Calculator Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product.

. These accelerated tests help pinpoint possible seal and burst strength faults leaks and film delamination in medical device and pharmaceutical packaging. Establishing the Shelf Life of Pharmaceutical Products. A product stored for stability at or near 15 C may have quite a different quality profile at its expiration date than a product stored at or near 30 C.
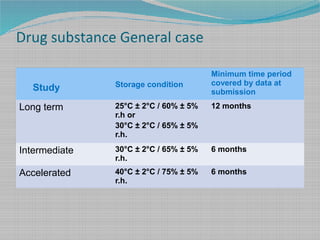
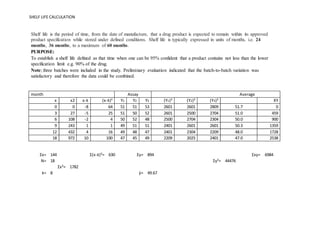
Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions. Shelf life calculator for pharmaceutical products. Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data.
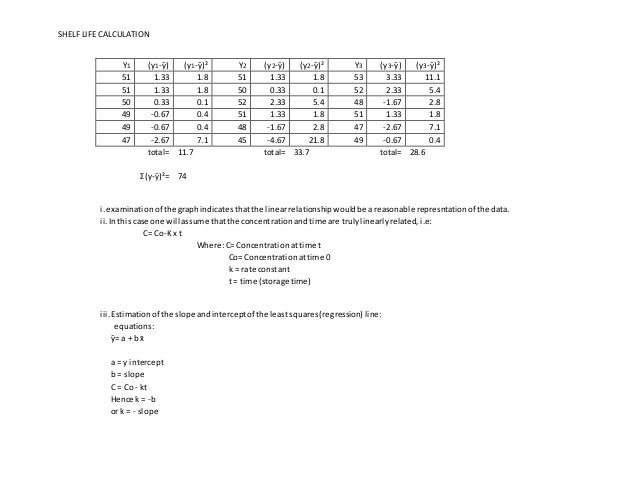
SHELF LIFE CALCULATION Shelf life is the period of time from the date of manufacture that a drug product is expected to remain within its approved product specification while stored under defined conditions. The batch is a single sample of the pharmaceutical products manufacturing process at. Stability as the extent to which a product retains within specified limits and throughout its period of storage and use the same properties and characteristics that it possessed at the time of manufacture.
Accelerated stability testing and shelf-life calculation. Minimum data of three batches are used to estimate the shelf life of pharmaceutical products. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration.
It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. 24 months for all presentations.
18 months 18 months and 9 months respectively for the three presentations. A pharmaceutical product is typically manufactured in batches. Each batch is distinguished by its own shelf life which can be called the true batch shelf life.
Shelf life calculator for pharmaceutical products. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. If no significant change is found in the accelerated conditions then shelf life would.
The tests are prominent in biomedical research pharmaceutical and. Type the target shelf life Days 2. 24 months 36 months to a maximum of 60 months.
The shelf-life of a drug product is the time that the average drug charac-teristic eg potency remains within an approved specification after manufacture. Shelf life is determined by the evaluation of whole stability data of the product. The quality within each step influences the quality of the resulting knowledge.
In the pharmaceutical industry drug products are usually manufactured in different batches. A batch is a fixed quantity of product for example 100000 tablets. Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by.
At least three distinct steps are necessary to gain knowledge and understanding of a product or process. Batch and Product Shelf Life. Initial shelf life.
Such comprehensive and common language is currently lacking from various guidelines which confuses implementation and impedes comparisons of different methodologies. The five new. Shelf life calculation of drugs.
After entering data you push button Check and calculator will show you if. Thats because as time goes on a products Sterile Barrier System can become altered as it faces stresses from time and the ambient environment. A batch is a fixed quantity of product for example 100000 tablets.
QUANTILE REGRESSION CALIBRATION. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field. Enter all three dates to the left to see the remaining shelf life.
On the Shelf Life of Pharmaceutical Products. The batch is a single sample of the pharmaceutical products manufacturing process at. Based on published information it.
Expiry with date in days in months or in years. New Drug Substances and Products presented at the FDA - GPhA Spring workshop in 2012 2013. Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product.
Q10 value can be changed however default is. The FDA requires testing of at least three batches preferably. The medical device industry in particular places a large emphasis on testing the shelf life of products.
Type desired TAA TRT Values. Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months. This can also be used to determine the shelf life of a product prior to purchase.
Depending on the response the confidence interval may be one or two sided. For the products stored at room temperature the assessment should be started from the occurrence of the significant change in product stored in accelerated conditions. This article proposes new terminology that distinguishes between different concepts involved in the discussion of the shelf life of pharmaceutical products.
This knowledge is critical across industries from consumer goods to pharmaceuticals. Also it is necessary to find the mark. For eg If the date of manufacture of a drug product is 5 Dec 2016 and the drug product has a shelf life of 2 years then the date of expiry shall be Nov 2018.
3 commercial batches manufactured at commercial scale met the approved stability specifications. Shelf life is typically expressed in units of months ie. Up to 10 cash back Batch and Product Shelf Life.

Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part I Youtube

Shelf Life Calculator For Composites And Other Materials

Stability Testing And Shelf Life Estimation

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Shelf Life Of Foods First Order Kinetics Example Youtube
Stability Testing And Shelf Life Estimation

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube

Long Term Food Shelf Life Food Storage Shelves Canned Food Storage Food Shelf Life

Stability Testing And Shelf Life Estimation

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

The Stability And Shelf Life Of Food
Shelf Life Calculation Of Drugs

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Wo2015122864a1 Accelerated Shelf Life Calculation Method Google Patents

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram